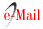Services on Demand
Article
Bookmark
TropIKA.net
On-line version ISSN 2078-8606
TropIKA.net vol.1 no.1 Jan./Mar. 2010
Dengue outbreak response: documented effective interventions and evidence gaps
Daniel PilgerI,*; Mark De MaesschalckII; Olaf HorstickI; Jose Luis San MartinIII
ISpecial Programme for Research and Training in Tropical Diseases (WHO/TDR), Geneva, Switzerland
IIPublic Health Doctor, Belgium
IIIPan American Health Organization, Panama
Date of database searches: February 2008.
ABSTRACT
BACKGROUND: 2.5 billion people, two-fifths of the world's population, are at risk from dengue with 50 million cases of dengue infection worldwide every year.
OBJECTIVES: To review the effectiveness of interventions employed during dengue outbreaks, to recommend an evidence-based strategy for the management of dengue outbreak response programmes, and to identify areas for further research.
METHODS: We searched for literature containing different terms for dengue (including dengue fever (DF), dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS)) combined with the terms "outbreak", "epidemic" and "intervention", "response", "control", "management" and "treatment" in the Cochrane Database of Systematic Reviews, PubMed, EMBASE, LILACS, WHO library database, grey literature, and through manual reference searching. Studies were included that measured the outcome of interventions implemented during outbreaks by entomological and/or human disease epidemiological parameters.
RESULTS: A total of 24 (out of 1134) studies met all the inclusion criteria. Different strategies in the organization of outbreak response were identified that clearly emphasized an intersectoral approach. Studies that managed the outbreak response by creating multidisciplinary response teams, including vector control teams working on a door-to-door basis, and studies that monitored and evaluated their activities, showed successful outbreak control. Combining interventions that use 1) vector control (elimination of larval habitats with community involvement; appropriate use of insecticides in and around houses) and 2) capacity training of medical personnel in combination with laboratory support, were crucial for the successful control of outbreaks. Spatial spraying of insecticides alone proved ineffective in achieving outbreak control and its usefulness in combination with other interventions remains doubtful.
CONCLUSION: Further research is needed that links the effectiveness of interventions used during the outbreak response to human disease epidemiology. However, available evidence indicates that, in order to achieve rapid control, the outbreak response must employ a multidisciplinary approach combined with monitoring and evaluation.
Introduction
Dengue is the most rapidly spreading viral vector borne disease worldwide (1). Approximately 2.5 billion people are living in areas with dengue transmission and an estimated 50-100 million infections occur annually (1, 2). In the last 50 years, the reported average annual incidence of dengue infection has increased 30-fold (1). Population growth, migration, poverty, the ineffective use of resources for prevention and control and rapid urbanization are the main factors fuelling the spread of dengue and causing recurrent epidemics (3-5).
In the absence of a vaccine and drugs, prevention of dengue fever (DF) and its more severe forms is of the utmost importance. Rapid responses to dengue outbreaks are needed in order to control the spread of the virus and to manage the high number of cases. A wide range of different interventions have been employed to meet these demands (6). An intersectoral approach with strong community communication and participation is the recommended best practice in the control of outbreaks. It is, however, unclear which interventions or combinations of interventions are effective.
To date, most studies on dengue outbreaks have focused on epidemiological surveillance (7) and vector control (8-10). Studies evaluating outbreak response are uncommon and are difficult to interpret, as they generally describe a wide range of interventions and interpret results in different ways. This review will identify research that provides empirical evidence on interventions implemented during dengue outbreaks and based on these existing data, we will make recommendations for dengue outbreak management and response.
Methods
Inclusion criteria
• Any study conducted during a dengue outbreak.
• Interventions specifically addressing the outbreak.
• Outcome of the intervention clearly. described and supported by empiric data (mentioned as text or figures).
Exclusion criteria
• Interventions not specifically addressing the outbreak e.g. general case management.
• Opinion papers, or general descriptions e.g. "decline in cases", "outbreak controlled".
Search strategy
MEDLINE, Excerpta Medica Database (EMBASE), the Cochrane Database for Systematic Literature Reviews (CDSR; published in The Cochrane Library), the Latin American and Caribbean Health Sciences Database (LILACS) and the World Health Organization (WHO) library database (WHOLIS) were searched until the end of July 2008. Different terms for dengue (including DF, dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS)) were combined with the terms "outbreak", "epidemic" and "intervention", "response", "control", "management" and "treatment". Search terms included MeSH and free-text terms. In addition, references from all included papers were hand-searched as well as grey literature, such as theses, WHO communications, conference papers and unpublished country evaluations. The search was restricted to English, French, Spanish, German and Portuguese literature. Dengue outbreak response was defined as the sum of measures specifically addressing a dengue outbreak, with the aim of reducing case fatality rates (CFR), the number of cases and or entomological parameters.
Quality assessment
The quality of included papers was assessed by the "hierarchy of study design" according to the Report for Undertaking Systematic Reviews on Effectiveness (11); all studies included were at evidence level 4 (observational studies without control groups) except for one study (12), which included a control group. The following study designs were evaluated: descriptive epidemiological studies (prospective or retrospective), before-and-after studies and evaluations using mixed methods. Due to the heterogeneity of the included studies and in the absence of an appropriate validated tool for quality assessment, these categories are merely descriptive and were not used to weight the studies.
Data extraction and synthesis
Data were extracted according to a pilot checklist adapted from the Cochrane Handbook for Systematic Reviews, February 2008 and agreed upon by two authors (DP and MD). To calculate inter-reviewer agreement Cohen's Kappa was used.
| Box 1 Entomological indices Breteau index (number of containers with immature stages per 100 houses) House index (number of houses containing immature stages per 100 houses) Container index (number of containers with immature stages per 100 containers with water) Pupae per person (number of pupae per individual in a given area). |
A data matrix containing the bibliographic information, study design, objectives, components of the outbreak response, purpose of the outbreak response, sectors involved in the organization of outbreak response, outcomes, attributes, and the conclusions was created (Annex 1). Missing information was obtained by contacting the authors of the study.
Assessment tools
The main objective of our study was to identify and assess different dengue outbreak strategies. Studies focusing on vector control entomological parameters, such as the ovipositioning rate, House-Index (HI), Breteau-Index (BI), Container-Index (CI) as well as pupae indices (Box 1) used alone (13, 14) or in combination with human epidemiological parameters, such as the number of dengue cases were included in our review (15-17).
Assessment of the case fatality rate during outbreak response was also considered (18-20).
We assessed the performance of interventions by comparing, categorizing and grouping the included studies, by considering: 1) how often an intervention was implemented, 2) the combination of interventions used, 3) the effectiveness as measured by outcome parameters and 4) the conclusions on the effectiveness of the interventions. Interventions and combination of interventions were considered successful if they reported a significant drop in the above-mentioned indices and if the authors reported on the effectiveness of the outbreak response. For disaccording results, the strategy was considered "uncertain successful" or "uncertain unsuccessful".
Outbreak response
According to the 1997 WHO global dengue guidelines (21) the major goals of outbreak management are to: i) curtail dengue transmission as rapidly as possible and ii) minimize the mortality associated with the epidemic. The results of this systematic review are grouped according to these two goals (although all studies used a combination of different interventions rather than a single one).
Results
The literature search identified a total of 1134 potentially eligible publications (duplicates not included) (Figure 1). The application of all inclusion and exclusion criteria by both authors resulted in a total of 24 articles and a Cohen's kappa of 0.62. Nine studies were from the Americas, 10 from Asia, 3 from Australia and 1 each from New Zealand and the Pacific region. Of these studies, 18 were published between 1990 and 2008, and the oldest study included was from 1945. The studies were grouped into three categories according to the main strategy of outbreak management (A: studies focusing on transmission reduction; B: studies focusing on mortality reduction; C: studies describing both) (Annex 1). The results of our assessment based on performance of intervention are summarized in Table 1.
Interventions aimed at reducing dengue transmission
Intersectoral approach and organization
As part of the outbreak response, 20 out of 24 of the studies employed an intersectoral approach to the planning, implementation and evaluation of control activities (Annex 1, Table 1).
In six studies from categories A and C, multidisciplinary outbreak response teams were set up during the outbreak (15, 22-26). These teams met weekly (26), collected information on ongoing control activities, identified areas of weakness and high priority for outbreak control (15, 23, 25).
The intersectoral approach was achieved through vector control teams composed of government agents, soldiers and members of the public (16, 27, 28). Involving the public had several advantages. Firstly, the recruitment of college students (17), volunteers (25, 29) or neighbourhood organizations (29) to support survey teams, allowed for wider and more intensified geographical coverage (25) thereby preventing official outbreak management personnel from becoming overworked (30). Secondly, the incorporation of neighbourhood associations or community leaders expanded community outreach and improved community acceptance of control measures (15). The latter was crucial, as reluctance of the community to participate in the control activities would have seriously compromised all efforts (31). In terms of law enforcement, incorporating police officers into survey teams was considered to be useful in increasing community participation in Taiwan and Singapore (15, 16).
In 13 studies, vector control teams operated on a door-to-door survey basis. They implemented control measures, collected data on clinical dengue cases, and larval habitats, and educated households (15-17, 28, 29, 31). This helped to intensify surveillance and to monitor the spread of the disease (16, 23, 28). Door-to-door visits were paid at different times of the day varying from once to twice a week, for a period of six weeks. Teng et al. concluded that repeated visits could also be important in increasing community awareness (16).
Community education and participation was achieved through various measures (Table 2), this is in keeping with the recognition that all sectors should be involved in disease control programmes. Three out of seven studies using interventions without community participation reported a success in controlling outbreaks (12, 13, 32), whereas 13 out of 14 studies with community participation reported successful control as a result of community participation (31). The statistical significance of this difference is not known. Additionally, all studies that incorporated community organizations within their outbreak management organizational structure achieved successful outbreak control (Table 1) and had a high level of community participation (17, 25).
Five of the 24 included studies monitored and evaluated all outbreak response activities (20, 23, 24, 27, 28). In these studies, a geographic information system (GIS) was used to map the control activities. Using GIS provided an added benefit of monitoring the spread of the disease (23, 33).
All studies that focused on vector control and reported successful outbreak control (except for Chan et al. (22)) implemented more than one of the above-mentioned interventions (Table 1). On the other hand, only two out of nine studies reported an unsuccessful outbreak control (or inability to control), when more than one of the above measures was applied.
Transmission reduction by insecticide spraying
Space spraying of insecticides was either performed peridomestically by vector control teams or by truck-mounted sprayers or by aerial spraying using aircraft. Two out of the three studies that used aerial spraying of insecticides describe unsatisfactory results. The intervention did not reduce the ovipositioning rate per household and only achieved a mean Aedes mortality rate of 55% (13). Furthermore, the intervention did not have a measurable effect on dengue incidence and adult mosquito landing rates rebounded within 48 hours (26). However, one study obtained "satisfactory results" that needed confirmation (23).
Terrestrial spraying using truck-mounted guns was an intervention described by four studies (23, 26, 28, 32). In Honolulu, truck-mounted spray guns working from one household to the next covered the area sufficiently, resulting in a sharp reduction of cases (28). In contrast, terrestrial spraying by truck-mounted spray guns proved ineffective in Suriname. The intervention was time-consuming, unfeasible for wide areas, and did not result in any change in dengue incidence (32). Both aerial and terrestrial spraying have the disadvantage of not reaching mosquitoes inside houses (13, 32). Chan et al. (22) concluded that truck-mounted spray guns would be ineffective in areas with high-rise buildings. One study in attempt to prevent mosquitoes from migrating from a treated to an untreated area, fumigated from the periphery of a municipality towards the centre (24). This intervention, combined with source reduction, achieved a drop in BI from 0.49 to 0.01. The study concluded that spraying has to take into account mosquito mobility. However, all the other studies (Table 1) that used space spraying as the only applied intervention for vector control concluded that it was ineffective.
Focal outdoor spraying or fogging was widely used. Tukuitongal et al. (34) observed a sharp reduction in cases after ULV (ultra-low volume) spraying of high-risk areas. Teng et al. (16) noted a similar reduction in cases and a pronounced decline in ovipositioning activity after the outdoor application of insecticides on mosquito resting sites in combination with source reduction. In agreement with this finding, Chan et al. after concentrating insecticide application on cryptic larval habitats or construction areas, achieved an Aedes PI reduction from 9.1% to 5% as well as a continued decline in human disease incidence (13, 15, 22, 23, 30).
Indoor spraying or fogging was generally considered an important measure in controlling adult vectors (13, 15, 16, 22, 23, 30, 33). When indoor application of insecticides was included in the outbreak response, it was used as a targeted intervention. One study used areas exceeding a HI of 2.0 and described an overall HI reduction from 5.8 to 2.4 after fogging with 3% malathion and Reslin 50E (15). In five studies, we observed that insecticides were applied to the premises and the surrounding areas of confirmed or suspected dengue cases (12, 16, 29, 30, 33, 35). Teng et al. (16) described a human case reduction from 31 per week to 1, after indoor spraying of Perdelta in 50 houses in the vicinity of a reported dengue case. However, Chadee et al. could not achieve a BI reduction below a transmission threshold of 5.0 after the application of 96% malathion and highlighted the importance of correct application (12). Studies from Australia considered selective indoor spraying with lambda-cyhalothrin or deltamethrin within a range of 100 to 300 meters of a dengue case crucial in confining the outbreak (30, 33, 35). Two of the studies from Australia emphasized the importance of 'ignition sites' (premises with a lot of travellers e.g. backpacker hostels) and targeting of 'dissemination venues' (sites with a high density of people, such as schools) for indoor spraying (30, 33).
Transmission reduction by environmental management and source reduction
From the 24 included studies, 15 used source reduction through elimination of possible larval habitats or application of larvicides (14-17, 20, 22, 24-28, 30-36). Two main methods of source reduction were used: "search and destroy teams" and community based source reduction.
Search and destroy teams worked in outbreak areas on a door-to-door basis, eliminating all larval habitats or, where elimination was impossible, they applied temephos or Bacillus thuringiensis israelensis (BTI) (25). Some teams also carried out the following interventions: inspection and repair of roof-top gutters (15), identification and treatment of cryptic larval habitats (33), promotion of sanitation (36), improvement of public drainage system and infrastructure (25), removal of old tires and installation of proper waste disposal (17). The two studies that focused only on adult mosquito reduction without any environmental management or source reduction (13, 32) could not report any change in entomological parameters in comparison to studies that included environmental management. Only four studies mentioned sanitation or improvement of infrastructure as part of the outbreak response (15, 20, 25, 36).
Of crucial importance for vector control through environmental management were community-based interventions (15, 17, 20, 22, 24-26, 28, 31). Out of the 24 included studies, 16 emphasized community based source reduction. Table 2 gives an overview of such strategies.
Transmission reduction by case management and restriction of public gatherings or movement of cases
One study reported the restriction of public gatherings to prevent people from becoming infected (34). A more frequently used approach was to quarantine infected individuals (18, 20, 24). Another study (20) described the use of boarding schools to temporarily hospitalize dengue cases and only referred these cases once the incidence had decreased. Kouri et al. considered this intervention crucial, as it removed highly infectious patients from the environment. In a study in India, to reduce transmission within the hospital, cases were treated under mosquito nets (18). As an alternative to hospitalization of dengue cases in Cuba, patients were advised to use mosquito nets at home (24).
Results of interventions aiming at reducing mortality
Reorganization of services and case management
In outbreak situations existing health care services can easily be overwhelmed by the sudden influx of patients. In the categories that aimed at mortality reduction (B and C), two out of five studies addressed this by redistributing doctors across the outbreak area and increasing the availability of primary care beds, e.g. by using schools. This intervention required a well-functioning case referral system for more severe cases (20, 27).
To plan, implement and evaluate the actions taken at hospital level and to liaise with local health authorities, outbreak control groups were established (18, 19). Hospital staff, such as physicians, nurses and vector control coordinators were included in the control team.
At the hospital level, one study (19) made use of an experienced team. The team assisted in the reorganization of clinical services, identified operational deficiencies, requested for additional resources, such as laboratory equipment, and reviewed case management. Staff received training on dengue and dengue treatment guidelines through formal lectures. Doctors also received "hands on training" during ward rounds by experienced staff. The laboratory was supported with additional equipment and staff were trained. This led to a drop in CFR from 12% to 3.6% (19). Three other studies (18, 20, 27) also trained doctors and supported the laboratory. These studies describe a decline in severity of the disease (20) and achieved a CFR of 1.23% (18). Additionally, the "hands-on" training of doctors and case report conferences were important tools for case management (18, 19). For non-hospital based physicians, telephone hotlines were set up with information on diagnosis and treatment (19) or in some cases the physicians were actively contacted (35).
Discussion
Dengue outbreak response
Transmission reduction
The results of our systematic review confirm the general recommendation of an intersectoral approach to dengue outbreak management (21, 37, 38). Intersectoral collaboration between different organizations was described in 12 out of the 24 included studies. Eleven out of these 12 studies reported successful results (Table 1). Five studies had a single intersectoral approach (two with multidisciplinary response teams [MRP], two with door-to-door teams [Dtd] and one with supervision). Use of MRPs (which involves several teams and thus more expertise) and supervision (which also involves different teams) had positive effects where as Dtd reported both positive and negative results. In summary, 16 out of the 24 analysed studies reported positive results when using intersectoral collaboration.
The involvement of different sectors in outbreak control teams was found from national to local levels as well as in operational levels. This approach is the most suitable, as there are many factors that influence outbreaks. Outbreaks present a considerable workload and economic burden, often overwhelming the capacities of a single sector (6). Extending the idea of an intersectoral approach to include international aid can be advantageous. In this review, two studies described good results with the incorporation of external aid by the CDC (26) and WHO (18), in order to deal with a situation that was overwhelming for local services. However, as diverse as intersectoral organization can be, this review identified that a key strategy for successful outbreak control relies on: a) good community communication, b) multidisciplinary response teams that incorporate public organizations, c) vector control teams operating on a door-to-door basis and d) monitoring and evaluation of all activities (Table 1).
In the control of outbreaks, timing is of utmost importance. When outbreak response is implemented near peak epidemic transmission it is unlikely to have any impact (5, 39). All of the studies included in this review implemented interventions relatively late during the outbreak or even after the outbreak had already reached its peak (27). This compromises the interpretation of the results as a decline in dengue cases could simply be due to the natural pattern of the outbreak and not as a result of an intervention. This calls for better surveillance systems. Nevertheless, while critically evaluating the success of the studies in containing the outbreak, a distinct pattern of combined interventions emerged. Studies describing inconclusive or negative outcomes implemented fewer interventions (mean number of 3) than studies with successful outcomes (mean number of 6). Out of 21 studies involving vector control, 19 made use of combined interventions (Table 1) and two studies concluded that only combined interventions were able to control the outbreak (22, 23). Furthermore, out of the four studies that reported a failure in controlling the outbreak (Table 1), 2 studies (12, 32) clearly focused only on a single intervention - spatial spraying of insecticides. This points to the importance of combined interventions when addressing an outbreak, as previously recommended (6), but also questions the use of spatial insecticide spraying. In fact, the value of this latter intervention in epidemic response remains in doubt with some studies considering spraying inferior to community based source reduction (17). However, selected spraying of premises (indoors and outdoors) targeting typical mosquito resting sites was considered important in containing the outbreak (16, 34) and a useful tool to control adult mosquitoes in a few studies (16, 33, 35). Spraying has to be implemented correctly and preferably following source reduction interventions (16). It could also be used pre-seasonally to avoid outbreaks (12). Good management and implementation through experienced teams is crucial to the success of spraying (12). Insecticide susceptibility should be monitored through bioassays (12, 16). Again, it is important to note, that the intervention seemed particularly effective when it was combined with other interventions such as source reduction (Table 1).
Furthermore, combined interventions have to include environmental management with reduction of larval habitats. As dengue outbreaks are also a consequence of uncontrolled urbanization (5), epidemic responses should investigate the cause of uncontrolled Aedes production i.e. abundant larval habitats, both natural and man-made. The results of the review support environmental management and source reduction interventions. Studies without these approaches failed to alter entomological indices (12, 13, 32), while studies that included environmental management through search and destroy teams that operated on a door-to-door basis, reported that this intervention had the most important impact on entomological indices and dengue transmission (14, 16, 17, 28).
For environmental management and source reduction community participation is crucial (17). Out of 16 studies, 11 concluded that community-based interventions were important in addressing the outbreak. This finding is supported by a systematic review which demonstrated that community based interventions are able to reduce entomological parameters (3).
It is important to note that such interventions rely on the willingness of the community to participate. Law enforcement can increase community participation (16, 22), but involving local leaders may be a more effective means of achieving community acceptance (15) as legal systems can vary.
In outbreak situations, hospitals can facilitate transmission of dengue, due to the high number of infected patients. Therefore, vector control must also focus on hospitals and the treatment of patients under mosquito nets. Arya et al. (18) concluded that fumigation and source reduction, combined with treatment of suspected and confirmed cases under mosquito nets, prevented the spread of dengue within a hospital.
As important as combined interventions may be to controlling outbreaks, one must consider the optimal combinations of different interventions may depend on the local setting of the outbreak. One Brazilian study (27) combined seven interventions and did not achieve satisfactory results, whereas other studies successfully controlled outbreaks with a combination of fewer interventions (16, 29).
Case management
Few of the studies retrieved focused on mortality reduction. Nevertheless, we are able to conclude from studies that included strategies to reduce mortality, that in outbreak situations local health services often lack the capacity and experience to deal with the high number of cases. These studies recommend that hospital staff should be trained and more attention should be paid to primary health care in order to reduce mortality (6, 21, 37, 38). Kalayanarooj et al. (19) concluded that hospital staff should be trained in early and late diagnosis and treatment. Additionally, laboratory facilities have to be strengthened. Again, this was best achieved by combining interventions such as training of doctors through "hands-on training" during ward rounds and case report conferences with laboratory support (19, 27).
Limitations of the review
The review mainly focused on published articles, probably leading to a selection bias as studies with poor outcomes could be less frequently published. In this review, only four studies which assessed health service management and reorganization reported negative results (12, 13, 31, 32). The publication bias was offset by collecting information from various sources other than scientific journals (grey literature) and including publications in different languages.
The inclusion of studies that only assessed the effect of their intervention through empiric data led to a higher selection of studies focusing on vector control and excluded narrative reports. Also, fewer qualitative studies dealing with the more operational aspects of outbreak management were included. On the one hand, the inclusion criteria were crucial for selecting studies that had comparable indicators for measurement. On the other hand, as the majority of studies are observational studies with various interventions and without a control group, information on the effectiveness does not provide a high level of evidence. It is therefore difficult to determine the effectiveness of a single intervention. Furthermore, interventions that worked in one country need not be applicable in a different setting as a variety of local aspects can influence the course of an outbreak.
Due to the heterogeneity and the different types of studies, it was not possible to assess statistically the overall performances of these studies. However, by comparing, categorizing and grouping all the interventions used and linking them to quantitative outcome indicators, we have been able to show patterns in the overall importance and effect (or lack of) of each combined strategy.
Finally, the application of a set of inclusion and exclusion criteria is liable to a higher degree of subjectivity, although the high Cohen's kappa value indicates that both authors (DP, MD) interpreted the articles in a similar way and the final interpretation was done by agreement of all authors of this study.
Conclusion
There is a lack of evidence on the effectiveness of dengue outbreak response interventions. In particular, data on mortality reduction and the effectiveness of improving infrastructure in outbreak response are limited. Further studies should address research pointed out in the review and highlighted in the summary boxes. Nevertheless, we can conclude that successful control of a dengue outbreak must include community participation, selective spraying of premises, and environmental management by search and destroy teams. Successful combinations of interventions that have been reported in the analysed studies are summarized in Box 2.
| Box 2 Summary of successful combinations of interventions for outbreak response I. Management of outbreak response:
II. Management of vector control services:
III. Management of health services:
|
| Summary boxes What has been learned from this review?
Implications for public health practice
Priority research areas
|
References
1. UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Report of the Scientific Working Group meeting on Dengue, Geneva, 1-5 October 2006. Geneva: World Health Organization; 2007. (Meeting report (UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases). [ Links ]
2. Gubler DJ (2006). Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp;277:3-16, discussion 16-22, 71-73, 251-253. [ Links ]
3. Heintze C, Garrido MV, Kroeger A (2007). What do community-based dengue control programmes achieve? A systematic review of published evaluations. Trans R Soc Trop Med Hyg;101(4):317-325. [ Links ]
4. Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R (2008). Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med;5(3):e68. [ Links ]
5. Gubler DJ (1998). Dengue and dengue hemorrhagic fever. Clin Microbiol Rev;11(3):480-496. [ Links ]
6. Rigau-Perez JG, Clark GG (2005). Como responder a una epidemia de dengue: vision global y experiencia en Puerto Rico. Rev Panam Salud Publica;17(4):282-293. [ Links ]
7. Runge-Ranzinger S, Horstick O, Marx M, Kroeger A (2008). What does dengue disease surveillance contribute to predicting and detecting outbreaks and describing trends? Trop Med Int Health;13(8):1022-1041. [ Links ]
8. Ashford DA, Savage HM, Hajjeh RA, McReady J, Bartholomew DM, Spiegel RA, Vorndam V , Clark GG , Gubler DG (2003). Outbreak of dengue fever in Palau, Western Pacific: risk factors for infection. Am J Trop Med Hyg;69(2):135-140. [ Links ]
9. Pontes RJ, Freeman J, Oliveira-Lima JW, Hodgson JC, Spielman A (2000). Vector densities that potentiate dengue outbreaks in a Brazilian city. Am J Trop Med Hyg;62(3):378-383. [ Links ]
10. World Health Organization. Dept. of Communicable Disease Prevention Control and Eradication., WHO Pesticide Evaluation Scheme. Space spray application of insecticides for vector and public health pest control : a practitioner's guide. Geneva: World Health Organization; 2003. [ Links ]
11. NHS Centre for Reviews and Dissemination (2001). Undertaking systematic reviews of research on effectiveness: CRD's guidance for carrying out or commissioning reviews. CRD's guidance for carrying out or commissioning reviews. York: NHS Centre for Reviews and Dissemination, University of York. [ Links ]
12. Chadee DD, Williams FL, Kitron UD (2005). Impact of vector control on a dengue fever outbreak in Trinidad, West Indies, in 1998. Trop Med Int Health;10(8):748-754. [ Links ]
13. Castle T, Amador M, Rawlins S, Figueroa JP, Reiter P (1999). Absence of impact of aerial malathion treatment on Aedes aegypti during a dengue outbreak in Kingston, Jamaica. Rev Panam Salud Publica;5(2):100-105. [ Links ]
14. Victor TJ, Malathi M, Gurusamy D, Desai A, Ravi V, Narayanasamy G, Anuradha L , Rani C , Krishnamurthy P (2002). Dengue fever outbreaks in two villages of Dharmapuri district in Tamil Nadu. Indian J Med Res;116:133-139. [ Links ]
15. Goh KT, Ng SK, Chan YC, Lim SJ, Chua EC (1987). Epidemiological aspects of an outbreak of dengue fever/dengue haemorrhagic fever in Singapore. Southeast Asian J Trop Med Public Health;18(3):295-302. [ Links ]
16. Teng HJ, Chen TJ, Tsai SF, Lin CP, Chiou HY, Lin MC Yang SY , Lee YW , Kang CC , Hsu HC , Chang NT (2007). Emergency vector control in a DENV-2 outbreak in 2002 in Pingtung City, Pingtung County, Taiwan. Jpn J Infect Dis;60(5):271-279. [ Links ]
17. Wang CH, Roam GD (1994). Dengue vector control in the urban environment of Taiwan. Gaoxiong Yi Xue Ke Xue Za Zhi;10 Suppl:S28-32. [ Links ]
18. Arya SC, Rajagopal S, Agarwal N, Kaushik M, Maheshwari P, Agarwal S, Singh K, George S (2004). Private sector hospital response to the 2003 dengue outbreak in the Indian capital metropolis of Delhi. Am J Infect Control;32(8):489-492. [ Links ]
19. Kalayanarooj S, Rimal HS, Andjaparidze A, Vatcharasaevee V, Nisalak A, Jarman RG, Chinnawirotpisan P , Mammen MP , Holmes EC , Gibbons RV (2007). Clinical intervention and molecular characteristics of a dengue hemorrhagic fever outbreak in Timor Leste, 2005. Am J Trop Med Hyg;77(3):534-537. [ Links ]
20. Kouri GP, Guzm'an MiG, Bravo JR, Triana C. Dengue haemorrhagic fever/dengue shock syndrome : lessons from the Cuban epidemic, 1981; 1989. [ Links ]
21. World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control: World Health Organization; 1997. [ Links ]
22. Chan KL, Ng SK, Chew LM (1977). The 1973 dengue haemorrhagic fever outbreak in Singapore and its control. Singapore Med J;18(2):81-93. [ Links ]
23. Coello D, Mazzarri M (1992). El control de vectores durante el brote epidÈmico de dengue en Venezuela noviembre 1989- marzo 1990. Cuad. Esc. Salud Publica;(58):3-22. [ Links ]
24. Guzman MG, Pelaez O, Kouri G, Quintana I, Vazquez S, Penton M, Avila LC ; Grupo Multidisciplinario para el Control de la Epidemia de Dengue 2001-2002 (2006). Final characterization of and lessons learned from the dengue 3 epidemic in Cuba, 2001-2002. Rev Panam Salud Publica;19(4):282-289. [ Links ]
25. Koh BK, Ng LC, Kita Y, Tang CS, Ang LW, Wong KY, James L, Goh KT (2008). The 2005 dengue epidemic in Singapore: epidemiology, prevention and control. Ann Acad Med Singapore;37(7):538-538. [ Links ]
26. Morens DM, Rigau-Perez JG, Lopez-Correa RH, Moore CG, Ruiz-Tiben EE, Sather GE, Chiriboga J, Eliason DA, Casta-Velez A, Woodall JP (1986). Dengue in Puerto Rico, 1977: public health response to characterize and control an epidemic of multiple serotypes. Am J Trop Med Hyg;35(1):197-211. [ Links ]
27. Barbosa da Silva J Jr, Siqueira JB Jr, Coelho GE, Vilarinhos PT, Pimenta FG Jr (2002) Dengue in Brazil: current situation and prevention and control activities. Epidemiol Bull;23(1):3-6. [ Links ]
28. Gilbertson WE (1945). Sanitary Aspects of the Control of the 1943-1944 Epidemic of Dengue Fever in Honolulu. Am J Public Health Nations Health;35(3):261-270. [ Links ]
29. Caraballo A, Hernandez J (1991). Brote de dengue en San Jose de Guaribe, Venezuela. Reporte preliminar. Revista do Instituto de Medicina Tropical de Sao Paulo;33(5):413-415. [ Links ]
30. Hanna JN, Ritchie SA, Phillips DA, Serafin IL, Hills SL, van den Hurk AF, Pyke AT, McBride WJ, Amadio MG, Spark RL (2001). An epidemic of dengue 3 in far north Queensland, 1997-1999. Med J Aust 2001;174(4):178-182. [ Links ]
31. Eamchan P, Nisalak A, Foy HM, Chareonsook OA (1989). Epidemiology and control of dengue virus infections in Thai villages in 1987. Am J Trop Med Hyg;41(1):95-101. [ Links ]
32. Hudson JE (1987). La campana de emergencia mediante rociamientos en volumenes ultrarreducidos contra ejemplares adultos de Aedes aegypti realizada en 1982 en Paramaribo, Suriname. Bol. Oficina Sanit. Panam;103(1):21-32. [ Links ]
33. Ritchie SA, Hanna JN, Hills SL, Piispanen JP, McBride WJH, Pyke A, Spark RL (2002). Dengue control in North Queensland, Australia: Case recognition and selective indoor residual spraying. Dengue Bulletin;26:7-13. [ Links ]
34. Tukuitonga CF, Maguire T (1988). An epidemic of type 3 dengue on Niue Island. N Z Med J;101(851):500-502. [ Links ]
35. Hanna JN, Ritchie SA, Richards AR, Taylor CT, Pyke AT, Montgomery BL, Piispanen JP , Morgan AK , Humphreys JL (2006). Multiple outbreaks of dengue serotype 2 in north Queensland, 2003/04. Aust N Z J Public Health;30(3):220-225. [ Links ]
36. Pelaez O, Guzman MG, Kouri G, Perez R, San Martin JL, Vazquez S, Rosario D, Mora R, Quintana I, Bisset J, Cancio R, Masa AM, Castro O, González D, Avila LC, Rodríguez R, Alvarez M, Pelegrino JL, Bernardo L, Prado I (2004). Dengue 3 epidemic, Havana, 2001. Emerg Infect Dis;10(4):719-722. [ Links ]
37. Organización Panamericana de la Salud. Dengeu y dengeu hemorrágico en als Américas: guías para su prenvenión y control. Washington D.C.: PAHO; 1995. [ Links ]
38. Regional Office for South-East Asia WHO. Prevention and control of dengue and dengue haemorrhagic fever: comprehensive guidelines. New Delhi: World Health Organization; 1999. [ Links ]
39. Gubler DJ (1989). Aedes aegypti and Aedes aegypti-borne disease control in the 1990s: top down or bottom up. Charles Franklin Craig Lecture. Am J Trop Med Hyg;40(6):571-578. [ Links ]
Submitted: January 2009; Accepted: June 2009; Published: December 2009
* Corresponding author: : Daniel Pilger, daniel.pilger@web.de
Annex 1 Data summary matrix of all included studies (n = 24)
Annex 2 Excluded studies